Fig. 4‑134 shows the δ34S and δ18O isotope signatures for vein celestite observed in the BOZ1 and STA3 boreholes. For comparison, isotope data for marine sulphate during different geological periods are also illustrated (Claypool et al. 1980), as well as δ34S and δ18O values of evaporites in the Bänkerjoch Formation and the Muschelkalk Group (Rick 1990, Pearson et al. 1991, Bernasconi et al. 2017). The vein celestite in all of the investigated samples shows δ18O versus δ34O outside the range of marine sulphate at all times at which seawater was potentially present in the region, indicating modification by sulphate reduction. This is further evidenced by the occurrence of pyrite as vein infill (Section 4.7.3). The slope of the resulting isotope enrichment in a plot of δ34S versus δ18O varies as a function of different factors (bacterial vs. abiotic reduction, open vs. closed system, effects of Rayleigh distillation) but mostly lies in the range of 0.25 – 0.4 (Pierre 1989, Clark & Fritz 1997). Fig. 4‑134 shows that such a slope fits reasonably well with the isotope data of celestite in the Malm Group, with Jurassic seawater as a likely source of the sulphate (i.e. internally sourced, pre-dating Eocene – Oligocene influx of meteoric water). One sample (marked with an asterisk in Fig. 4‑134) falls off this general trend by showing a distinctly lower δ18O value. The sulphate of this vein celestite potentially originates from Burdigalian seawater associated with the deposition of the Upper Marine Molasse Group. A similar hypothesis was proposed by de Haller et al. (2011) for vein celestite in the Malm Group of the Oftringen (OFT) borehole.
For vein celestite in the Opalinus Clay, a slope of 0.25 – 0.4 in the δ34S versus δ18O plot also fits reasonably well the isotope data, however, with sulphate originating in the underlying evaporites of the Keuper. Based on similar trends, Mazurek & de Haller (2017) also proposed Keuper-sourced sulphate for vein celestite in the Opalinus Clay at the Mont Terri rock laboratory. This does not necessarily imply advective communication between the Keuper units and the Opalinus Clay, it could result from diffusive exchange over long timescales. Present-day porewaters in the Opalinus Clay – Lias interval show increasing sulphate concentrations with increasing depth (e.g. Nagra (ed.) 2023a, Dossier VIII), thus, this concentration gradient makes upward diffusion of sulphate a plausible scenario.
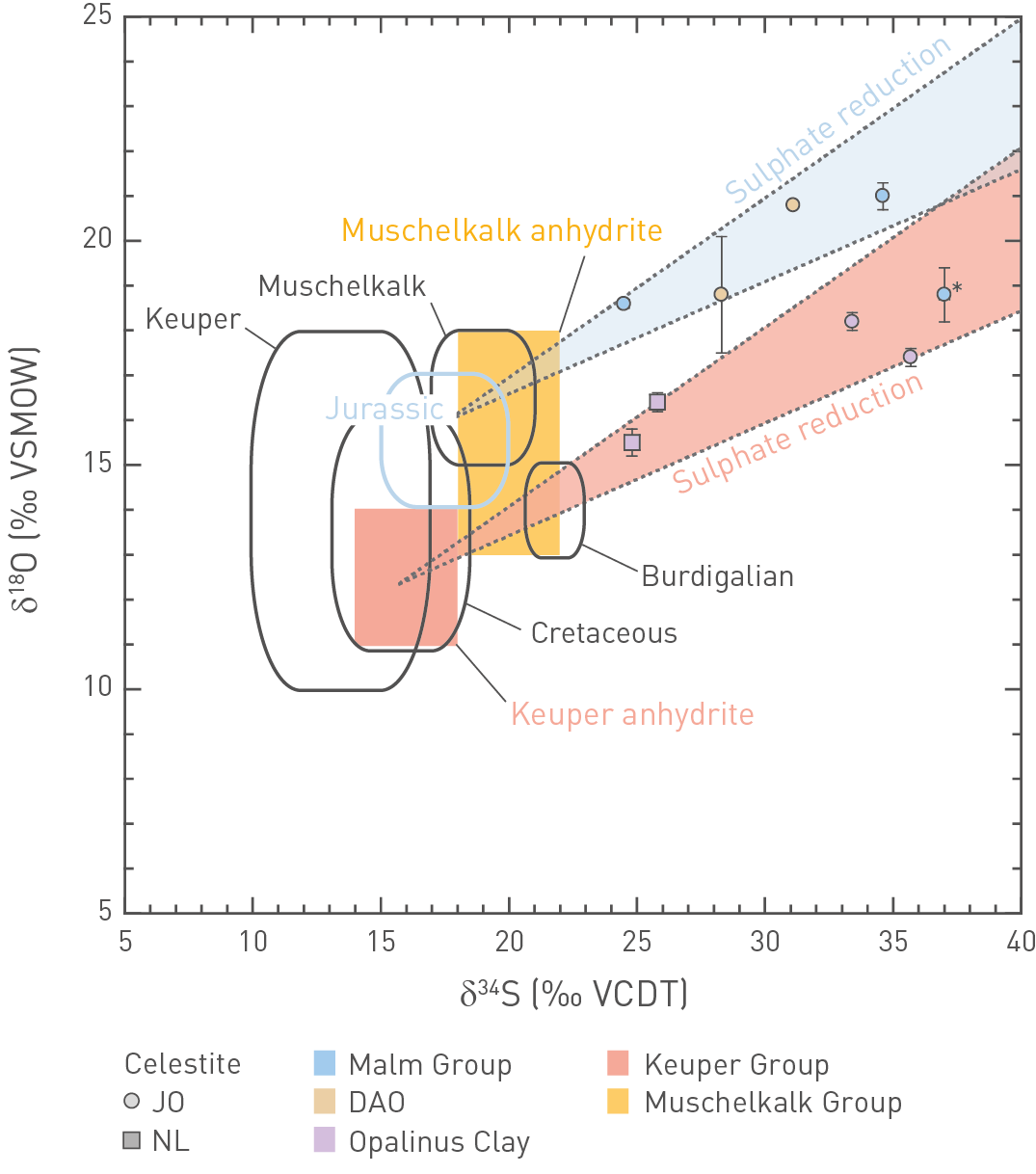
Fig. 4‑134:δ18O and δ34S values for vein celestite observed in boreholes in Northern Switzerland
Solid lines represent ranges for marine sulphate during different geological periods (Claypool et al. 1980). Data for anhydrite in the Gipskeuper and Muschelkalk are from Rick (1990), Pearson et al. (1991) and references therein and Bernasconi et al. (2017). DAO: Dogger Group above Opalinus Clay. The two fans bordered by dotted lines indicate the trends of sulphate reduction (Pierre 1989, Clark & Fritz 1997). These trends fit the isotope data of vein celestite reasonably well, suggesting Jurassic (and Burdigalian) seawater as the source of sulphate for vein celestite in the Malm and DAO, whereas vein celestite in Opalinus Clay seems to be sourced from Keuper anhydrite. Celestite occurrences at ZNO were generally too small to be analysed.

